Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and differentiate between periods and groups in the periodic table, recognizing their significance in organizing elements.
ii. Explain the concept of increasing atomic number as the basis for arranging elements in periods and groups.
iii. Identify the characteristics and trends of elements within periods and groups, highlighting their similarities and patterns.
iv. Apply the knowledge of periods and groups to predict and explain the chemical properties of elements.
v. Appreciate the periodic table as a valuable tool for understanding and classifying elements.
Introduction
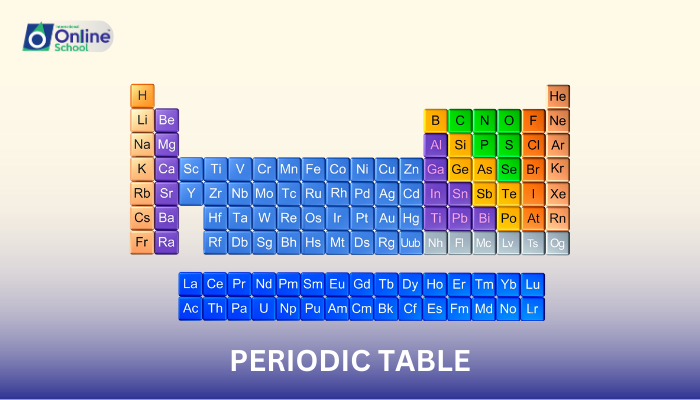
The periodic table, a cornerstone of chemistry, serves as a comprehensive arrangement of elements, providing a wealth of information about their properties and behavior. Within this intricate arrangement, periods and groups play crucial roles in organizing and classifying elements based on their similarities.
i. Periods: A Horizontal Parade of Elements
Periods, the horizontal rows in the periodic table, represent a sequence of elements with increasing atomic number. Moving from left to right across a period, the atomic number increases by one, and the number of protons and electrons increases accordingly.
Elements within a period exhibit similar chemical properties due to their identical valence electron configurations. The outermost electrons, known as valence electrons, are primarily responsible for an element's chemical behavior.
ii. Groups: A Vertical Column of Similarities
Groups, the vertical columns in the periodic table, represent a collection of elements with similar outer electron configurations, also known as valence electron configurations. Elements within a group share similar chemical properties due to their analogous valence electron arrangements.
Moving down a group, the number of electrons increases, resulting in elements with increasing atomic numbers. Despite this increase, the valence electron configuration remains consistent within a group, leading to similar chemical properties.
iii. Trends in Periods and Groups
Periods and groups reveal distinct trends in the properties of elements:
Atomic Radii: Atomic radii, the measure of an atom's size, generally decrease across a period from left to right due to the increased nuclear charge.
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally increases across a period from left to right due to the increased nuclear charge.
Electronegativity: Electronegativity, the measure of an atom's ability to attract electrons, generally decreases down a group.
iv. Predicting Chemical Properties
Understanding periods and groups allows us to predict and explain the chemical properties of elements:
Valence Electron Behavior: Valence electron configurations determine the type of chemical bonds an element can form.
Reactivity: Reactivity of elements is often related to their position in the periodic table, with elements on the left and right exhibiting contrasting behaviors.
Periodic Trends: The periodic table serves as a powerful tool for predicting and explaining trends in chemical properties.
Periods and groups, the fundamental organizing principles of the periodic table, provide a framework for understanding the similarities and trends in the properties of elements. By delving into the concepts of periods and groups, we gain insights into the chemical behavior of elements, enabling us to predict and explain their interactions and reactions.